Placental Health Study
The Placental Health Study (PHS) is a prospective study initiated by Dr. John Kingdom, the creator of the Placenta Clinic at Mount Sinai Hospital, in order to examine the relationships between pregnancy outcomes and placental function in first-time mothers. This study uses information which is already available (such as maternal clinical history and blood tests performed routinely in antenatal management) and combines it with a “placental health ultrasound” to look at possible correlations between placental function and neonatal outcomes. Below, you will find relevant information on background and rationale for the creation of the study, progress during the pilot phase and possible implications of this work, as well as how to find out more!
Background
Healthy placental function
The placenta (click here for “Placenta 101”), a transient organ present only during pregnancy, connects the developing baby to the mother by invading and attaching to the inside wall of the uterus. It ensures proper fetal nourishment by facilitating gas, nutrient and waste exchange between fetal and maternal blood circulations. Although maternal and fetal blood systems never mix, this exchange is made possible by passive and active transport of molecules across the placental villi, or terminal projections from the maternal side of the placental disc. The space between the villi, called the intervillous space, is filled with maternal blood which arrives here via the uterine spiral arteries and brings oxygen and nutrients for the baby. Following the exchange across the villi, maternal blood, now deoxygenated and saturated with metabolic waste products from the fetus, exits the intervillous space via maternal uterine veins. On the fetal side, baby’s blood leaves the fetus via umbilical arteries and passes through thin-walled arterioles where the nutrient and gas exchange occurs. Once fetal blood undergoes this exchange, it leaves the placenta, now full with oxygen, via the umbilical vein to return to the fetus. In a healthy pregnancy, maternal spiral arteries transform from constricted to fully-dilated blood vessels, with the goal of ensuring optimal blood delivery into the intervillous space. The effectiveness of this transformation can be assessed with the pulsatility index (PI), a measure of uterine artery constriction: a high PI value indicates persistent constriction of maternal arteries, and, thus, compromised nutrient delivery to the growing baby.
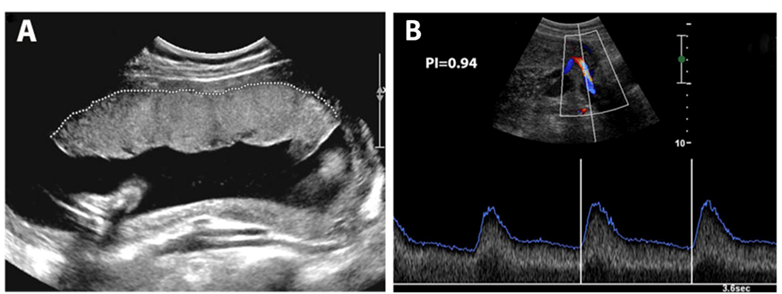
In order to function properly, the placenta must meet the following conditions: (1) an adequate size and (2) a well-established blood supply from the mother’s uterine arteries. Placental size is assessed during an ultrasound screen which determines the shape and size (panel A below shows a placenta with a normal shape and size). Blood supply is assessed using Doppler ultrasound of the uterine arteries (panel B shows a normal uterine artery Doppler with a low PI of 0.94). We term the combination of placental shape/size and uterine artery blood flow assessments using the ultrasound technique as the “placental health ultrasound.”
Placentally-mediated complications of pregnancy
Although the majority of pregnancies proceed normally resulting in healthy term deliveries, a small subset of women may experience complications of pregnancy. An impaired placental function (seen by abnormal ultrasound results) may indicate that some of these problems are placentally-mediated. These placentally-mediated complications present mostly after the baby has become “viable,” that is, after 24 weeks’ gestation and are referred to as perinatal complications of pregnancy.
Major complications are:
- Pre-eclampsia (PE) – characterized primarily by high maternal blood pressure and elevated protein levels in the urine
- Intrauterine growth restriction (IUGR) – poor growth of the developing baby
- Stillbirth – loss of baby’s heartbeat before birth
- Placental abruption – separation of the placenta from uterine wall
How is the placenta abnormal in these conditions?
- The most common placental abnormalities are:
- Smaller than normal placentas (chorion regression)
- Narrow or diseased blood vessels feeding into the intervillous space (decidual vasculopathy)
- Damaged and/or infarcted areas of placental tissue (dead areas of tissue)
- Invasion of the placenta by the mother’s immune system (inter-villositis, villitis)
- Abnormal umbilical cord (improper placental insertion, over-coiling)
Our hospital is very active in placental pathology research. Click here to learn more about the different types of placental pathology that cause severe pre-eclampsia or IUGR. Click here to learn more about the causes of placental infarction. Click here for a recent book chapter review on this subject by Dr. Kingdom.
What are the risk categories of pregnancy complications for pregnant women?
In broad terms, the pregnant population can be divided into 3 groups in terms of risk of placental insufficiency disorders:
- Low risk: multiparous women, in current pregnancy with the same partner, now less than 35 years of age, with previous normal full-term pregnancy (pregnancies) with vaginal delivery and without any significant medical problems. For these women, screening for placental complications is not justified.
- Intermediate risk: apparently healthy nulliparous women for whom screening for placental insufficiency may be justified. This is the population of women targeted in our Placental Health Study.
- High risk: nulliparous women with complex medical problems (ex. metabolic syndrome) and multiparous women with complex pregnancy histories (ex. history of IUGR and/or pre-eclampsia, gestational diabetes, stillbirth). These women are directed to receive care with obstetricians specializing in high-risk pregnancies (maternal-fetal medicine specialists) who can be found in programs such as our Special Pregnancy Program at Mount Sinai Hospital. Diagnostic testing is indicated for women in this group to ensure proper fetal monitoring and a timely delivery.
Why are first-time mothers at a higher risk of placentally-mediated complications?
The placenta, like the baby, is made from equal genetic contributions of the mother and father. Since the placenta has 50% of its genetic material from the father, it tries to hide the differences between mother and father by suppressing expression of the paternal genes responsible for “tissue rejection” (click here to learn more about this complex subject). Sometimes this suppression does not work well. Women embarking on their first pregnancy (nulliparous women) are “untested” for tissue compatibility (the ability to mount an effective decoy response), in comparison with pregnant women who have had a successful healthy pregnancy with the same partner (multiparous women). Interestingly, pregnant women who have had one or more early miscarriages with the same partner have a lower risk of placental complications compared to women who had not miscarried previously (click here for more information).
Rationale and purpose of the Study
As described in the previous section, women embarking on their first pregnancy are at an intermediate risk of developing placentally-mediated pregnancy complications. Therefore, in order to decrease their risk and help monitor their pregnancy progression, we suggest introducing a “placental health ultrasound” scan into their antenatal care, with the aim of catching complications as early as possible and customizing their health care plans to ensure a healthy pregnancy and a successful delivery of the baby.
The Placenta Health Study aims to determine if the “placental health ultrasound” performed at 20+0-22+6 weeks of pregnancy, in combination with other easily-available information (ex. first trimester blood results, maternal medical history, and pregnancy outcomes), can reliably identify the small subset of women in their first ongoing pregnancy that are at risk of significant placental complications.
Who can participate?
Women who fit the following criteria are welcome to participate:
Inclusion Criteria:
- Nulliparous women (no previous pregnancy > 20 weeks)
- Singleton pregnancy (one fetus)
- With blood work done (IPS or FTS plus MSS testing)
- Antenatal care and anticipated delivery at Mount Sinai Hospital
Exclusion Criteria:
- Multiple pregnancy (e.g. twins or triplets)
- Recurrent vaginal bleeding during pregnancy
- Major fetal abnormality detected at the 19 week ultrasound scan
- Short cervix (<2.0cm) detected at 19 week ultrasound scan
- Ruptured membranes
What constitutes a “placental health ultrasound” exam?
The placental health ultrasound exam is performed between 20+0 and 22+6 weeks of gestation. This scan comprises of two parts: (1) assessment of placental shape and size which looks at placental length, thickness and insertion into the uterine wall; and (2) real-time Doppler ultrasound assessment of blood flow through maternal uterine arteries which looks at pulsatility of these blood vessels.
Before the placental health ultrasound exam:
You are required to obtain a photocopy of your antenatal 1 record (AN1) from your obstetrician, family physician or midwife affiliated with Mount Sinai Hospital and bring it to the Placental Health Study office (Room 3-904 in the Ontario Power Generation building) where you will meet Dr. Kingdom’s administrative secretary, Ms. Millie Xue. She will ask you to complete a brief 5-minute questionnaire (click here to view this form if you prefer to complete it at home). Assuming that you meet the eligibility criteria, she will book your appointment for the placental health ultrasound examination. Please note: you must have already had your detailed 19 week fetal anatomy “level 2” ultrasound examination the results of which were normal.
Placental health ultrasound examination:
The examination will be done by our RDMS-certified senior research sonographer Viji Ayyathurai at the same location (3rd floor of the Ontario Power Generation building). The ultrasound examination will take approximately 20 minutes and usually there is no wait-time for this appointment. Images of your placenta during the real-time 2D ultrasound will be obtained, after which a 3D sweep of the placenta will be done. These are part of the placental shape/size assessment. Next, maternal blood flow to the placenta will be examined using the Doppler ultrasound for both right and left uterine arteries. The panel of images below shows images from a placental health examination in normal (A and B) and abnormal (C and D) scans. If you bring a USB key or blank CD to your exam, we will provide you with a digital 3D image of your baby similar to the one seen on the left! (assuming the baby is in a good position to obtain a picture)
You will be told about the results of your exam immediately. The Placenta Clinic uses electronic records to keep track of your ultrasound results, and a copy of the scan will be faxed to your health care provider. Additionally, a copy can be provided to you upon your request.
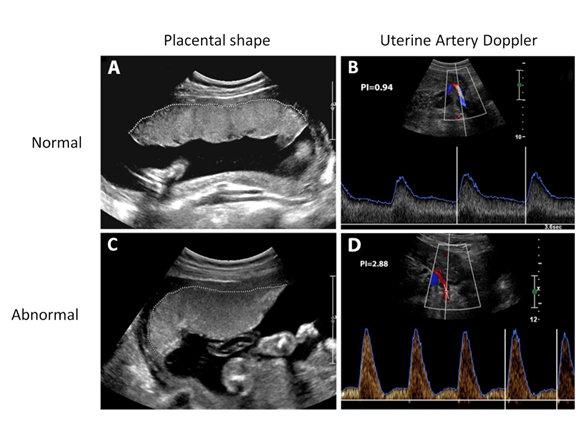
Normal ultrasound results:
It is anticipated that 49 out of 50 women will have a “normal” result during this placental health ultrasound exam. A normal result predicts a low risk of a placental problem, equivalent to the level of a healthy “low risk” multiparous woman; this means that your risk of developing hypertension, impaired fetal growth and/or premature birth is very low.
Abnormal ultrasound results:
On the contrary, it is anticipated that 1 out of 50 women will be given an “abnormal” or “screen-positive” result. An abnormal result constitutes abnormalities in both placental morphology AND uterine artery Doppler reports. Abnormal placental pathology will be defined as a small (maximum length 95th percentile for gestational age.
What does an abnormal result mean?
Women with an abnormal result are at higher-than-average risk of a placental problem. We predict that their risk of preterm delivery
What happens once I am told I have an abnormal placental ultrasound exam?
If your ultrasound examination is abnormal, the following scenario will occur:
- The ultrasound images will be reviewed in 24-48 hours by placental specialists Dr. Kingdom or Dr. Windrim.
- A verified report will be faxed to your care provider and he or she will be contacted by Dr. Kingdom or Dr. Windrim.
- You will be given a consultation appointment at the Placenta Clinic at Mount Sinai Hospital within 2 weeks to meet Dr. Kingdom or Dr. Windrim and for a repeat ultrasound examination.
- At this time, you will have a chance to discuss with the doctors the subsequent course of your neonatal care, including the introduction of frequent ultrasounds to monitor your baby’s growth at the Placenta Clinic. All records obtained during ultrasound examinations at Mount Sinai Hospital will be shared with your care provider.
How are “screen-positive” women managed?
The following interventions will be available to women who have abnormal results during the placental health ultrasound exam:
- Targeted education about the symptoms and signs of pre-eclampsia as well as more frequent antenatal clinic visits to detect the early signs of this disease and the option of home blood pressure monitoring;
- Use of anti-hypertensive pills or “blood pressure lowering drugs” to control elevated blood pressure and thereby prevent unsafe elevations in blood pressure;
- Ultrasound assessment of fetal growth and well-being by measuring blood flow to the baby’s brain and other parameters. These ultrasound examinations can be incorporated into a program of antenatal visits which would initially take place every 2 weeks, increasing to weekly if any evidence of growth restriction is found;
- Admission to our hospital for more intensive monitoring of blood pressure of the mother, and for more frequent ultrasound or heart-beat tests (the non-stress test, NST) of fetal well-being. Our Hospital is a perinatal centre, designed to provide excellent care to babies that may need to be born prematurely as a result of placental or other complications;
- Administration of 4 intra-muscular injections (into the thigh) of steroids (dexamethasone, 6mg) every 12 hours, to make the baby’s internal organs, especially the lungs, stronger in preparation for the need to deliver prematurely (before 34 weeks);
- If delivery is required before 32 weeks, we may additionally give the mother an intravenous infusion of magnesium sulphate (MgSO4) to elevate baby’s magnesium levels at birth to protect the developing brain;
- For pregnancies that continue successfully towards term, a pro-active plan to deliver (for example by induction of labour) at 37-40 weeks will avoid the risk of stillbirth due to placental insufficiency. Click here for the current level of evidence to support this intervention;
- By taking a pro-active approach to assessing the mother and baby, we expect to avoid the potential complications that could arise from failure to make the correct diagnosis;
- If the mother was not aware of her risk of pre-eclampsia, she may underestimate the severity of a medical emergency that headaches, vomiting and abdominal pain could indicate
- If the health care provider was not aware of her risk of poor baby growth (IUGR), the developing baby is at risk of stillbirth
Can we take your placenta after you deliver?
After you deliver your baby, we would like to send your placenta for a pathology exam. We believe this step is critical to test the accuracy of the concept of “placental health” assessment. Many studies have assessed these tests to predict pregnancy outcomes, though they have never included examination of the placenta, for two reasons. First, many hospitals do not have a major commitment to placental pathology. We know from our research that placental pathology is a highly-valuable guide to future pregnancy decisions, typically surpassing the value of blood tests. Our hospital has the focus and commitment to provide high-quality placental pathology services. The second factor is cost. Our current grant does not cover the placental pathology costs, but we are hoping to secure the necessary costs (at least $100/case) from future grants and grateful donations.
Capturing the placenta for pathology assessment in every woman screened in the Study is important for the several reasons:
- Some women develop pre-eclampsia due to maternal clinical risk factors, ie, not due to placental dysfunction. These factors were identified by the SCOPE study (click here for more information).
- Not all cases of pre-eclampsia/IUGR are associated with findings of a pathology characterized by small and infarcted placentas. We predict, however, that this common underlying pathology will be effectively detected by the placental health ultrasound (giving a “true positive” result).
- By performing placental pathology in the entire screened cohort, we can evaluate the contributions from other types of serious placental pathology that in theory develop after the 20+0-22+6 week ultrasound examination and may be very difficult to detect, as they are essentially only visible under the microscope. These are fortunately rare and include:
- Maternal immune cell invasion (inter-villositis, villitis of unknown etiology, histiocytosis)
- Massive peri-villous fibrin deposition (maternal floor infarction)
- Fetal thrombotic vasculopathy (including cord accidents)
- Finally, it is possible that a certain level of “placental damage” is tolerated successfully by the mother and her developing baby. If this is true, we may be able to adjust the cut-off values for our screening ultrasound, so as to limit the proportion of “false-positive” women who are potentially harmed (anxiety, increased visits, interventions such as earlier induction of labour).
What are the associated risks and benefits?
There are no direct risks to participating in the study, as the only addition to standard health care protocol is the placental ultrasound, which is a non-invasive technique, no different from the standard of care for high-risk pregnant women in the Special Pregnancy Program. The benefits of this study are most pronounced for women identified at-risk of placentally-mediated complications. With early detection, these women will be offered better antenatal care plans to carefully monitor baby’s growth and plan a timely delivery. This has the potential to prevent stillbirth and/or intrauterine fetal demise, as well as ensure the mother’s wellbeing by routinely measuring (and managing) her blood pressure and other vital functions. Furthermore, information obtained throughout the course of this study will benefit pregnant women in the future, in the event that the proposed placental health ultrasound scan is sufficiently accurate to identify a small subset of women at risk of developing placentally-driven pregnancy complications.
What are the implications of this study?
The results of this study will help determine what screening methods, or what combination of screening methods, are best at detecting placental dysfunction. These results could influence current Ontario Prenatal Screening program and the Society of Obstetricians and Gynaecologists of Canada (SOGC) clinical practice guidelines of growth restricted infants, and may improve screening, diagnosis, and management of women at high risk of severe placental dysfunction and placentally-mediated complications of pregnancy.
How have we done so far? Our progress!
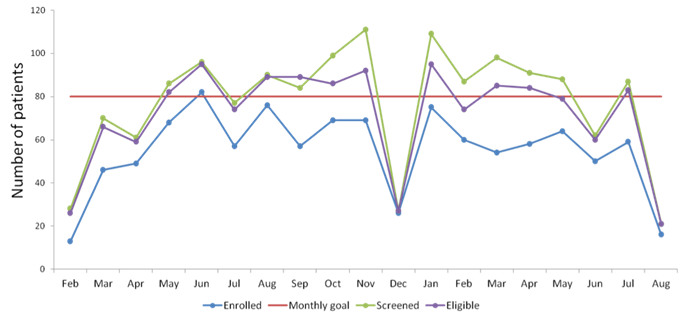
We began the pilot phase of this program in February of 2012. To date, we have been very successful. We are currently following more than 1,000 women who have already delivered or are planning to give birth at Mount Sinai Hospital. We aim to recruit 5,000 women over the next 5 years. As shown below, we currently recruit 60-80 participants per month. This research has been very well received by our patient population, with approximately 80% of eligible women agreeing to participate.
The pilot phase of our study is complete, and we are in the process of analyzing the results. Updates on what these results show are coming soon!
Frequently asked questions
Can I still have a homebirth?
Yes. Your Midwife will bring your placenta to Mount Sinai Hospital if you are a participant in this study.
I want to collect cord blood at my baby’s birth. Is this still possible?
Yes. We will collect the cord blood for stem cells immediately after delivery of the baby, while the placenta is still attached to the inside of your body. After this, we can send the placenta to pathology.
I would like to take my placenta home with me after I give birth. Is this possible?
Yes. We strive to be inclusive of all cultures. We will simply record the clinical outcomes at delivery, weigh and describe your placenta. It will not go through a detailed pathology exam.
What happens if the placental pathology is to some degree abnormal, but I had a normal term delivery?
All abnormal pathology is reported to Dr. Kingdom. He will contact your care provider if he feels that the findings merit a discussion prior to your next pregnancy. A mutually-convenient time will be arranged for a meeting.
Is 3D ultrasound safe?
Yes. The energy output is low and the duration of exposure to obtain the image is quite short. We, therefore, believe that 3D ultrasound is as safe as normal 2D ultrasound, which is a standard part of prenatal care.
Contact us to find out more
If you would like more to find out more about the Placental Health Study, please contact the persons listed below.
Natasha Milligan, BSc
Project Coordinator
Phone: (416) 586-4800 ext. 6419
Email: Natasha.Milligan@sinaihealth.ca
Dr. John Kingdom, MD
Phone: (416) 586-4800 ext. 8764
Email: John.Kingdom@sinaihealth.ca
Funding and REB permission
This pilot study is funded for two years (2012-2014) by a grant to Dr. Kingdom from the Innovation Fund at Mount Sinai Hospital and University Health Network. The funds support the salaries of part-time staff: a booking secretary, a skilled ultrasonographer, a project coordinator and a research assistant.
The project received approval by Mount Sinai Hospital Research Ethics Board (REB) and is being renewed annually.
